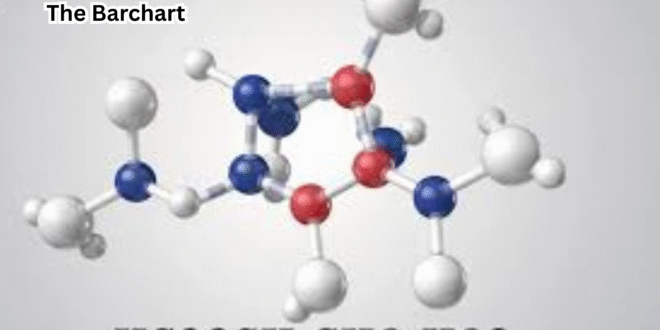
hcooch ch2 h2o reaction involves the interaction between formic acid (HCOOH), a methylene group (CH2), and water (H2O), representing a significant transformation in organic chemistry.
Understanding the structure of this reaction sheds light on how molecules behave, bond, and convert under various conditions.
Scientifically, this reaction is important in fields like synthetic chemistry, where controlled reactions are essential for developing new compounds.
The applications of the HCOOH CH2 H2O reaction span laboratory experiments, industrial processes, and environmental studies, particularly in understanding reaction pathways and energy dynamics.
By decoding this reaction, chemists can innovate safer, more efficient processes while also deepening their understanding of molecular interactions at the core of organic transformations.
Understanding the Chemical Reaction: HCOOH + CH2 → H2O Explained
The chemical equation HCOOH + CH2 → H2O represents a simplified form of a reaction involving formic acid (HCOOH) and a methylene group (CH2), resulting in the formation of water (H2O).
hcooch ch2 h2o this reaction is likely part of a broader organic mechanism where CH2 acts as a reactive intermediate or radical, possibly in a substitution or elimination reaction.
Formic acid, hcooch ch2 h2o being the simplest carboxylic acid, plays a crucial role in organic synthesis due to its reactivity.
When it interacts with a methylene species, water is often released as a byproduct in condensation-type reactions.
Understanding this basic reaction is essential for grasping more complex chemical processes in both lab and industrial settings, especially in synthetic organic chemistry.
What Happens When HCOOH Reacts with CH2 and Produces H2O?
When HCOOH reacts with CH2 and forms H2O, it typically indicates a dehydration or condensation reaction.
In organic chemistry, such interactions often lead to the formation of larger molecules or intermediates.
The methylene group (CH2) is highly reactive, especially in radical or carbene form.
It can insert itself into bonds or react with acidic protons such as those found in formic acid.
The release of H2O suggests that a new carbon-carbon or carbon-oxygen bond may be forming.
This transformation is key in various synthetic pathways, including polymer formation and organic molecule construction.
Such a mechanism could be applicable in fuel synthesis or chemical manufacturing, where controlling water byproduct is essential for purity and efficiency.
HCOOH CH2 H2O Reaction: Chemical Equation and Its Importance
The hcooch ch2 h2o reaction, though written simply, symbolizes more than just a formula—it embodies an essential chemical transformation.
Formic acid (HCOOH) and methylene (CH2) reacting to produce water (H2O) reflects a basic unit of organic chemistry: bond formation and elimination.
This reaction could serve as a model for understanding how reactive intermediates like CH2 can drive chemical change, especially in controlled synthesis environments.
The importance of this reaction lies in its versatility; it can occur under various conditions, including thermal, catalytic, or photochemical environments.
Additionally, hcooch ch2 h2o reaction showcases how water elimination is often used to shift reaction equilibrium in favor of product formation, a fundamental concept in chemical kinetics and thermodynamics.
Applications of the HCOOH CH2 H2O Reaction in Organic Chemistry
The hcooch ch2 h2o reaction has several applications in organic chemistry.
It can be a foundational step in carbon-chain elongation or the creation of more complex organic compounds.
One of the key areas where this reaction is utilized is in the synthesis of esters or aldehydes, where water is a common byproduct.
Additionally, understanding the reactivity of methylene with formic acid helps chemists manipulate and design better catalysts, especially in hydrogenation and carbonylation reactions.
This reaction can also be used to simulate reaction conditions in academic and industrial research.
Whether in drug development or materials science, this simple reaction model provides insights that help guide larger synthetic strategies.
Is the HCOOH CH2 H2O Reaction Reversible? A Detailed Analysis
Reversibility in chemical reactions depends on multiple factors such as thermodynamics, the stability of products, and reaction conditions.
The hcooch ch2 h2o reaction is generally not reversible under standard conditions due to the elimination of water, which drives the reaction forward.
However, under specific conditions such as in the presence of water or with temperature control, a reverse pathway might be theoretically possible.
For example, hydrolysis reactions often reverse esterification by reintroducing water.
Yet, due to the unstable nature of CH2 as a standalone species, reversing this exact reaction in a lab setup is rare and highly controlled.
This makes the hcooch ch2 h2o reaction a good example of kinetic control versus thermodynamic control in chemistry.
Breaking Down the Molecules: HCOOH CH2 H2O Structure and Bonds
To understand the hcooch ch2 h2o reaction, it’s important to analyze the structure and bonding of each molecule.
HCOOH (formic acid) consists of a carboxylic acid group with a highly polar O-H and C=O bond, making it reactive in proton transfer and nucleophilic attack.
CH2, often a reactive intermediate, has two unpaired electrons that make it highly unstable and reactive, typically existing in radical or carbene forms.
H2O, the product, is a stable, polar molecule with strong hydrogen bonding capability.
The transformation from these starting materials to water typically involves the formation and breaking of covalent bonds, often facilitated by catalysts or heat.
This molecular understanding is essential for both theoretical chemistry and applied reactions.
HCOOH CH2 H2O in Real-World Chemical Processes
The hcooch ch2 h2o reaction, or its variations, plays a role in several real-world chemical processes.
In industrial synthesis, formic acid is used in fuel cells and as a reducing agent, where reactions with methylene-type intermediates can generate useful products.
In environmental chemistry, understanding how methylene interacts with carboxylic acids like HCOOH helps in modeling the behavior of pollutants.
This seemingly simple reaction also finds relevance in polymer chemistry, where dehydration reactions are crucial for chain formation.
Thus, HCOOH CH2 H2O represents more than just a classroom example; it has direct relevance in multiple scientific and industrial domains.
Thermodynamics of the HCOOH CH2 H2O Reaction
Thermodynamically, the hcooch ch2 h2o reaction tends to be exergonic, meaning it releases energy and favors product formation.
The generation of water as a byproduct often pulls equilibrium toward the products, especially in closed or dry reaction environments.
Enthalpy and entropy changes are critical in determining reaction spontaneity.
The removal of water shifts equilibrium according to Le Chatelier’s Principle, promoting further reaction progress.
Catalysts can be used to lower activation energy and control the pathway, ensuring high yield.
The reaction may also be sensitive to temperature; heating can drive off water, enhancing conversion rates.
Understanding these thermodynamic aspects is key to optimizing laboratory and industrial reactions involving HCOOH and CH2.
Lab Experiment: How to Demonstrate the HCOOH CH2 H2O Reaction
Demonstrating the hcooch ch2 h2o reaction in a lab setting requires careful planning and controlled conditions.
Although methylene (CH2) is unstable, it can be generated in situ using diazomethane or other precursors.
Safety precautions are vital, as CH2 intermediates can be hazardous.
The setup often includes a reflux system, an inert atmosphere (like nitrogen), and accurate temperature control.
By documenting the water produced and analyzing the remaining products, chemists can verify the reaction mechanism.
This experiment serves as an excellent teaching tool for organic chemistry students learning about reactive intermediates and dehydration reactions.
Environmental Impact of the HCOOH CH2 H2O Reaction Products
The environmental impact of the hcooch ch2 h2o reaction is minimal if the process is controlled and scaled appropriately.
Formic acid is biodegradable and considered less harmful compared to other industrial acids.
Water, as the primary byproduct, is environmentally benign.
However, concerns may arise during large-scale production, especially if the CH2 source involves toxic intermediates like diazomethane.
Proper containment and neutralization systems are essential to avoid environmental release.
Additionally, this reaction could be part of greener synthesis methods that avoid hazardous solvents or generate recyclable byproducts.
As industries move toward sustainable chemistry, understanding and mitigating the environmental footprint of even simple reactions like hcooch ch2 h2o is crucial.
Conclusion
The reaction involving hcooch ch2 h2o may appear simple at first glance, but it encapsulates a wide array of chemical principles, from reaction mechanisms to practical applications in organic synthesis.
This process showcases the interaction of formic acid (HCOOH) with a methylene group (CH2), leading to the formation of water (H2O) and possibly other intermediates, depending on the conditions.
The hcooch ch2 h2o reaction helps us understand vital aspects of organic chemistry such as functional group behavior, energy transfer, and molecular stability.
Beyond laboratories, this reaction has implications in chemical manufacturing, environmental chemistry, and academic research.
By studying it in depth, scientists gain insights into broader reaction patterns and develop better strategies for utilizing such transformations in real-world scenarios.
In essence, the hcooch ch2 h2o reaction is a gateway to comprehending fundamental and advanced chemical processes.